

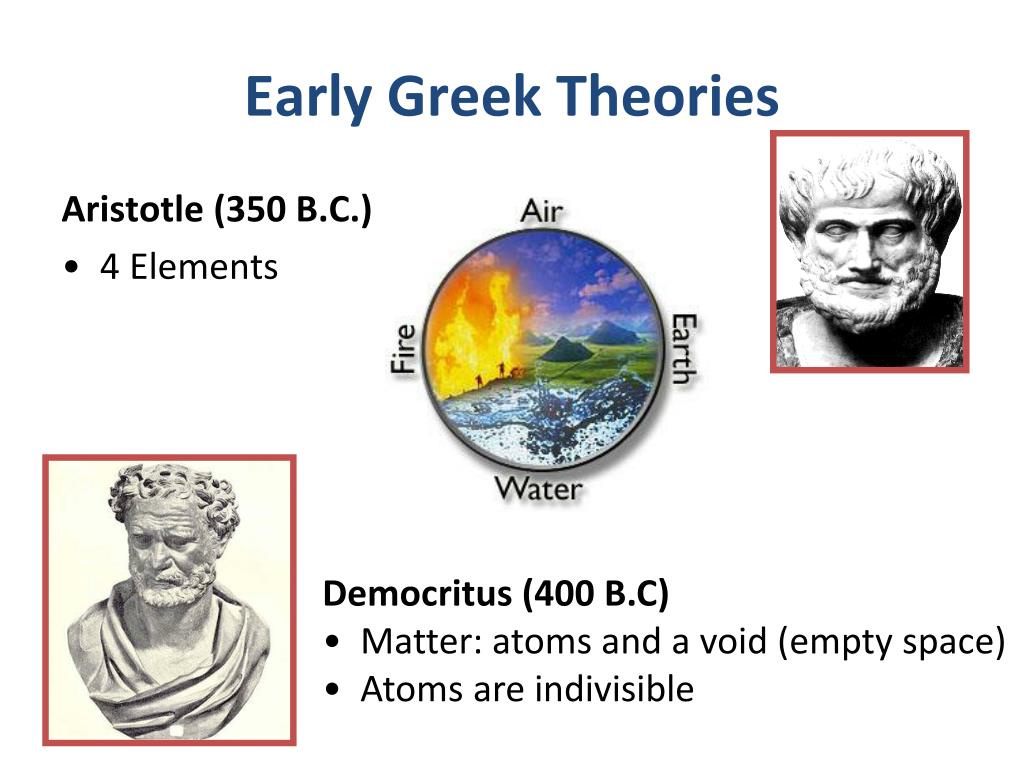
Atoms are indivisible they are the elementary grains of reality, which cannot be further subdivided, and everything is made of them. ‘Sweetness is opinion, bitterness is opinion heat, cold and colour are opinion: in reality only atoms, and vacuum,’ said Democritus. They have no weight, no colour, no taste. Atoms have no qualities at all, apart from their shape. Space is without limits it has neither an above nor a below it is without a centre or a boundary. The idea of Democritus’s system is extremely simple: the entire universe is made up of a boundless space in which innumerable atoms run.


Leucippus and Democritus came up with this idea. An idea was needed, a great idea, a grand vision, to grasp the hidden order of the world. It was a first germ of physics, rough and elementary, but in the right direction. Anaximenes of Miletus had imagined this substance could compress and rarefy, thus transforming from one to another of the elements from which the world is constituted. They had conceived of a kind of elementary substance from which everything was made. They had become convinced that the variety of natural phenomena must be attributable to something simple, and had tried to understand what this something might be. What Leucippus and Democritus had understood was that the world can be comprehended using reason. ‘Who is there whom we can compare with him for the greatness, not merely of his genius, but also of his spirit?’ asks Cicero. ‘The most subtle of the Ancients,’ Seneca called him. Democritus, the great pupil who wrote dozens of works on every field of knowledge, was deeply venerated in antiquity, which was familiar with these works. Together, these two thinkers have built the majestic cathedral of ancient atomism. On his arrival in Abdera, Leucippus founded a scientific and philosophical school, to which he soon affiliated a young disciple, Democritus, who cast a long shadow over the thought of all subsequent times. He wrote the book The Great Cosmology, in which he advanced new ideas about the transient and permanent aspects of the world. The traveller’s name was Leucippus little is known about his life, but his intellectual spirit proved indelible. It was to be a crucial journey for the history of knowledge. The Bohr atomic model later replaced the Rutherford model.According to tradition, in the year 450 BCE, a man embarked on a 400-mile sea voyage from Miletus in Anatolia to Abdera in Thrace, fleeing a prosperous Greek city that was suddenly caught up in political turmoil. Rutherford linked this motion to the orbit of planets around the sun. The angle of deflection from the particles also showed that there was most likely a strong positively charged nucleus in the middle of the atom with negatively charged particles circling around it. This made the most sense, since it explained why so few particles were hitting the gold foil. As a result, Rutherford created a theory that stated that most of an atom was empty space. Only about one in 8,000 was deflected away into the surrounding detecting screen. Through this experiment, Rutherford determined that the vast majority of the particles he fired at the gold foil passed right through it. The detecting screen had zinc sulfide in it to allow Rutherford to detect the presence of particles after they passed through the filtering gold foil. Ernest Rutherford’s gold foil experiment involved a particle emitter, a round detecting screen with a slit in it and a slip of gold foil in the middle.


 0 kommentar(er)
0 kommentar(er)
